| |
Advanced hemodynamic monitoring |
| |
Introduction |
| |
Hemodynamic monitoring is a cornerstone of care for the hemodynamically unstable patient.It is routinely used in the operating room during high-risk surgery. |
| |
Hemodynamic monitoring comes from an understanding of the pathophysiology of the process being treated, such as heart failure or hypovolemic shock.
|
| |
New Concepts in Hemodynamic Monitoring |
| |
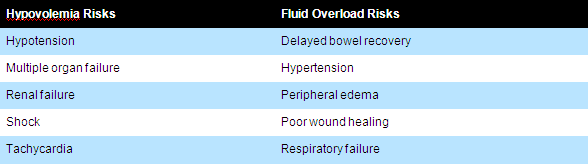
Circulatory shock is defined by decreased ability of blood flow to meet the metabolic demands of the body. Four basic groups of circulatory shock can be defined: Hypovolemic, Cardiogenic, Obstructive, and Distributive.
|
| |
Tissue hypo-perfusion is common in all forms of shock (with the possible exception of hyperdynamic septic shock). Because specific types of circulatory shock require different therapies and target end-points of resuscitation, defining the cardiovascular state is important in determining both treatment options and their goals.
|
| |
Much of the rationale for hemodynamic monitoring resides at this level.
|
| |
 |
| |
Hemodynamic Profiles in Shock |
| |
Certain combinations of hemodynamic findings allow the etiology of circulatory shock to be defined using this nosology.
|
| |
| Class of Shock |
CVP |
PAOP |
CO/CI |
SVR |
| Cardiogenic |
CVP |
PAOP |
CO/CI |
SVR |
| Hypovolemic |
CVP |
PAOP |
CO/CI |
SVR |
| Hyperdynamic septic |
CVP |
PAOP |
CO/CI |
SVR |
| Hypodynamic septic |
CVP |
PAOP |
CO/CI |
SVR |
|
| |
The ultimate goal of management of shock is to maintain “Tissue Perfusion” which is monitored by “Adequate Oxygen Delivery”.
|
| |
Oxygen demand > Oxygen delivery |
| |
Review of Basics: |
| |
Oxygen Delivery (DaO2) = CaO2 x CO x 10 = 1000 ml/min |
| |
Arterial Oxygen Content (CaO2) = (0.0138 x Hgb x SaO2) + (0.0031 x PaO2) = 20.1 ml/dl |
| |
Cardiac Output (CO) = SV x HR =4-8 L/min |
| |
Mixed venous (SvO2) and central venous oxygen saturations (ScvO2) reflect the balance between oxygen requirement and oxygen delivery, and thus may be used to assess the adequacy of tissue oxygenation. |
| |
Venous oximetry allows the critical estimation of the global oxygen (O2) supply-demand ratio and can be gained from mixed (SvO2) and central venous blood (ScvO2). |
| |
 | SvO2 - True mixed venous oxygen saturation (60-80%) |
 | ScvO2 - Central venous oxygen saturation (70%) |
 | VO2 – Consumption of oxygen (200-250ml O2/min) |
 | DO2 – Delivery of oxygen (950- 1150 ml O2/min) |
|
| |
Pulmonary artery catheterization allows obtaining true mixed venous oxygen saturation |
| |
(SvO2) while measuring central venous oxygen saturation (ScvO2) via central venous catheter reflects principally the degree of oxygen extraction from the brain and the upper part of the body. |
| |
(For details of Venous Oximetry refer Anaesthesia Pearls Feb 2013- http://www.isakanyakumari.com/february-1-2013.php) |
| |

|
| |
Continuous SvO2/ScvO2 Monitoring: |
| |

|
| |
A decrease in SvO2 and ScvO2 represents an increased metabolic stress, either DO2 does not increase in such a way to cover an increased VO2, or DO2 drops because of decrease in either arterial O2 content, cardiac output, or both. The magnitude of the decrease indicates the extent to which the physiological reserves are stressed.
|
| |
How do we measure SvO2% & ScvO2%? |
| |
Swan Ganz Pulmonary Artery Catheter (Old method) |
| |
Advanced Technology Catheters: |
| |
 | CCO (Continuous CO) |
 | CCOmbo (Continuous CO + Venous Oximetry) |
 | CCOmbo/VIP |
 | CCOmbo/EDV |
 | SvO2 |
 | CCO/CEDV |
 | CCOmbo/CEDV |
 | CCOmbo/CEDV/VIP |
|
| |
| Parameters |
Derived Information |
| SvO2 (Mixed venous Oxygen Saturation) |
Tissue Oxygenation |
| CEDV (Continuous End Diastolic Volume) |
Preload |
| SVR (Systemic Vascular Resistance) |
Afterload |
| CCO (Continuous Cardiac Output) |
Contractility |
| SV (Stroke Volume) |
Contractility |
| REVF (Right Ventricular Ejection Fraction) |
Contractility |
|
| |
Normal Hemodynamic Values |
| |
| SVO2 |
60-75% |
| Stroke volume |
50-100 mL |
| Stroke index |
25-45 mL/M2 |
| Cardiac output |
4-8 L/min |
| Cardiac index |
2.5-4.0 L/min/M2 |
| MAP |
60-100 mm Hg |
| CVP |
2-6 mm Hg |
| PAP systolic |
20-30 mm Hg |
| PAP diastolic |
5-15 mm Hg |
| PAOP (wedge) |
8-12 mm Hg |
| SVR |
900-1300 dynes.sec.cm-5 |
|
| |
These parameters can be used for Early Goal-Directed Therapy Treatment Protocol: |
| |
 |
| |
How to assess volume responsiveness? |
| |
 | Stroke Volume Variations (SVV) |
 | Pulse Pressure Variations (PPV) |
 | Systolic Pressure Variations (SPV) |
|
| |
Volume Responsiveness was defined as increase in CO by 15% or more, by 500 ml fluid bolus or by Passive Leg Raising. |
| |
The volume responsiveness can also assessed by CVP-MAP Relationship or CVP vs. EDV/EDVI. |
| |
New Generation Monitors uses Stroke Volume Variations, Pulse Pressure Variations and Systolic Pressure Variations for assessment of volume responsiveness. |
| |
Stroke Volume Variation - FloTrac Vigileo: |
| |
(For details of SVV refer Anaesthesia Pearls Feb 2013-http://www.isakanyakumari.com/february-1-2013.php) |
| |

|
| |
SVV can be used as a tool for volume responsiveness in low CO states. |
| |
SVV > 13% = Volume Responsive. |
| |
“SVV and PPV are more effective indicators for Volume Responsiveness than static indicators of preload (CVP, PAOP).” |
| |
Limitations: |
| |
 | Patient needs to be on 100% Controlled Mechanical Ventilation. |
 | Spontaneous Ventilation |
 | Arrhythmias can affect SVV. |
|
| |
Hemodynamic Monitoring |
| |
Static Hemodynamic Monitoring: |
| |
 | Heart Rate |
 | Blood Pressure ( Noninvasive vs. Invasive) |
 | Central Venous Pressure. |
 | The pulmonary artery catheter (PAC) |
|
| |
Functional hemodynamic monitoring: |
| |
 | Volume challenge |
 | Passive leg raising |
 | Changes in central venous pressure during spontaneous breathing |
 | Changes in left ventricular output during positive pressure ventilation |
|
| |
Advanced hemodynamic monitoring: |
| |
 | Cardiac Output Measurement – CCO, CCI, SV, SVI, SVV |
 | Tissue Oxygen Saturation Measurements- SvO2 or ScvO2 |
 | Echocardiography (TTE, TEE.) |
 | Thoracic Bioimpedance |
 | Thoracic Bioreactance |
 | Endotracheal CO Monitor |
 | The NICO System (Fick’s Principle for CO2) |
 | Esophageal Doppler |
|
| |
 |
| |
Clinical caveats for hemodynamic variables |
| |
| Type of hemodynamic variable |
Parameter |
Comments |
| Solitary |
Blood pressure |
Hypotension is always pathological |
| |
Central venous pressure (CVP) |
CVP is only elevated in disease |
| |
Pulmonary artery occlusion pressure (Ppao) |
Ppao is the back-pressure to pulmonary blood flow |
| |
Cardiac output |
There is no normal cardiac output, only an adequate or inadequate one |
| |
Mixed venous oxygen saturation (SvO2) |
Decreasing SvO2 is a sensitive but nonspecific marker of cardiovascular stress |
| Dynamic |
Volume challenge |
Positive response defined as an increase in any of blood pressure, CVP, Ppao, cardiac output and/or SvO2, or a decrease in heart rate |
| |
Echocardiographic analysis of vena cavae collapse
during positive pressure inspiration identifies CVP <10 mmHg if it detects
|
Complete inferior vena caval collapse a
>36% collapse in superior vena cavaa
|
| |
Defining preload responsiveness |
≥13% pulse pressure variation during positive pressure ventilationa
>1 mmHg decrease in CVP during spontaneous inspirationb
|
| a - Requires a fixed tidal volume of 6–8 ml/kg and complete adaptation to the ventilator |
b- Requires a spontaneous inspiratory effort greater than –2 mmHg to be valid.
(Source: Critical Care December 2005 Vol 9 No 6 Pinsky and Payen) |
|
| |
Central venous pressure (CVP) |
| |
CVP can reflect a volume increase in RA pressures or decrease in RV contractility, can be both. Need to be monitored in conjunction with other monitors. (CVP&MAP) |
| |
The main limitations of CVP monitoring: |
| |
 | It does not allow to measure cardiac output |
 | It does not provide reliable information on the status of the pulmonary circulation in the presence of left ventricular dysfunction. |
|
| |
Measurement of Cardiac Output: |
| |
Thermo Dilution (TD) |
| |
 | Dilution of Temperature (Cold NS) |
 | Area Under the Curve (AUC) |
 | Pulmonary Artery Catheter ( PACs) or |
 | Newer Generation CVL w sensors |
 | Intermittent vs. Continuous TD |
|
| |
Dye/Indicator Dilution |
| |
 | Same Technique ( Dilution of dye or indicator instead of NS) |
 | Dye(indocyanine green) vs. Indicator( Lithium) |
 | A line vs. CVL , no need for PACs. |
|
| |
Arterial Pulse Pressure Analysis |
| |
Pulmonary Artery Catheters (PACs): |
| |
| Both Thermo Dilution and Dye/Indicator Dilution require PACs to calculate Cardiac output. |
| The KEY advantage is simultaneous measurements of other hemodynamic parameters: |
 | Cardiac Output(CO) |
 | Pulmonary Artery Pressures ( PAD, PAOP) |
 | Left Sided Filling |
 | SvO2% |
|
|
 |
|
| |
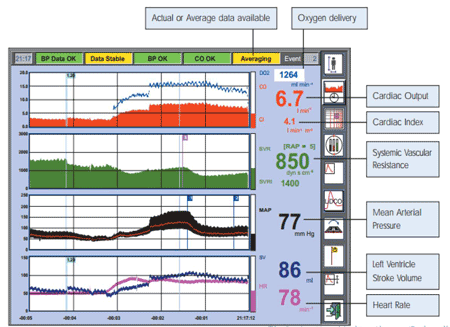
The highlighted parameters provided by the Swan-Ganz Adva nced Technology pulmonary artery catheter DELIVER the most comprehensive view of oxygen flow and consumption: |
| |
 |
| |
Source: Swan-Ganz Brochure, Edwards Critical Care Education |
| |
Other ways of measuring CO by dilution technique: |
| |
Transpulmonary Thermodilution Methods |
| |
 | PiCCO & PiCCO2 (Pulsion Medical Systems) |
 | VolumeView (Edwards Life Sciences) |
 | Intermittent vs. Continuous TD |
|
| |
Lithium Dilution Technique |
| |
 | LiDCO /LiDCOplus/LiDCOrapid ( LiDCO limited) |
|
| |
Ultrasound Indicator Dilution
|
| |
 | COstatus (Transonic Systems, Inc.) |
|
| |
COstatus (Transonic Systems, Inc.)
|
| |
With a single bolus of saline, provides key hemodynamic parameters including the following: |
| |
Cardiac Function
|
| |
 | Cardiac Output (CO) |
 | Cardiac Index (CI) |
 | Stroke Volume Index (SVI) |
 | Total Ejection Fraction (TEF) |
 | Systemic Vascular Resistance Index (SVRI) |
|
| |
Blood Volumes
|
| |
 | Total End Diastolic Volume Index (TEDVI) |
 | Central Blood Volume Index (CBVI) |
 | Active Circulation Volume Index (ACVI) |
|
| |
Shunt Detection
|
| |
 | Identifies shunts with direction of flow |
|
| |

|
| |
Source: http://www.transonic.com/ICU/COstatusSetup |
| |
Less Invasive Methods for CO Calculation
|
| |
They are Arterial Waveform Analysis - Waveform Derived CO Measurements. |
| |
| “BP is a product of SV (CO) and Vascular Resistance.” |
| Advantages: |
 | Non invasiveness |
 | Works through an already existing aline catheter |
 | Continuous CO monitoring |
|
| May require calibration for arterial compliance and resistance: |
 | Lithium Dilution ( LiDCO) |
 | Thermodilution (VolumeView, PiCCO) |
|
|
 |
|
| |
 |