| |
Safe Extubation |
| |
Dr.K.Sudarshan
Consultant Anaesthesiologist
ORTHO ONE hospital, Coimbatore
|
| |
Tracheal extubation is a critical step during emergence from general anaesthesia. It is not simply a
reversal of the process of intubation because conditions are often less favourable than at the start of
anaesthesia. At extubation, there is a transition from a controlled to an uncontrolled situation.
Anatomical and physiological changes, compounded by time pressures and other constraints, contribute
to a situation that can be more challenging for the anaesthetist than tracheal intubation. Although the
majority of problems following extubation are of a minor nature, a small but significant number have
serious consequences, including hypoxic brain injury and death.
|
| |
All anaesthetists will at some point experience difficulty with or after tracheal extubation. Whilst
tracheal intubation has been extensively studied, including the prediction, mechanism and incidence of
difficult tracheal intubation coupled with established guidelines, tracheal extubation has received little
attention. Despite tracheal extubation being recognised as a situation that can be potentially life
threatening, there is little literature and guidelines are based on limited scientific evidence and mainly
expert opinion.
|
| |
Data from the UK suggest that respiratory complications are common at extubation and during recovery
In the fourth National Audit Project (NAP4) of the Royal College of Anaesthetists and the DAS, major
airway complications occurred during emergence or in recovery in approximately one third of the
reported cases relating to anaesthesia. Closed-claims data from the US have demonstrated morbidity
and mortality associated with extubation . Following the publication of the ASA guidelines for
management of the difficult airway, there was a statistically significant reduction in airway claims arising
from injury at induction of anaesthesia. However, claims arising from injury intra-operatively, at
extubation and during recovery did not change. Death or brain injury was more common in claims
associated with extubation and recovery than those occurring at the time of induction of anaesthesia
|
| |
Problems at extubation: why is extubation hazardous? |
| |
Data from the UK suggest that respiratory complications are common at extubation and during recovery
In the fourth National Audit Project (NAP4) of the Royal College of Anaesthetists and the DAS, major
airway complications occurred during emergence or in recovery in approximately one third of the
reported cases relating to anaesthesia. Closed-claims data from the US have demonstrated morbidity
and mortality associated with extubation . Following the publication of the ASA guidelines for
management of the difficult airway, there was a statistically significant reduction in airway claims arising
from injury at induction of anaesthesia. However, claims arising from injury intra-operatively, at
extubation and during recovery did not change. Death or brain injury was more common in claims
associated with extubation and recovery than those occurring at the time of induction of anaesthesia
|
| |
Exaggerated laryngeal reflexes |
| |
Breath holding, coughing and bucking (a forceful and protracted cough that mimics a Valsalva anoeuvre) are physiological responses to airway stimulation and are associated with increases in arterial blood pressure, venous pressure and heart rate. |
| |
Laryngospasm |
| |
It is a protective exaggeration of the normal glottic closure reflex, and is produced by stimulation of the superior laryngeal nerve. Laryngospasm is often triggered by the presence of blood, secretions or urgical debris, particularly in a light plane of anaesthesia. Nasal, buccal, pharyngeal or laryngeal irritation, upper abdominal stimulation or manipulation and smell have all been implicated in the aetiology of laryngospasm. Clinical experience suggests that intravenous anaesthesia using a propofol-based technique is associated with a lower incidence of complications related to exaggerated airway reflexes, and there is some evidence to support this. |
| |
Typically, laryngospasm causes signs of upper airway obstruction (including stridor) that can precede complete airway obstruction and requires an immediate response (Appendix 1A). If not relieved promptly, laryngospasm may result in post-obstructive pulmonary oedema (also known as negative pressure pulmonary oedema) and hypoxic cardiac arrest. The equivalent response in the lower
airway is bronchospasm.
|
| |
Reduced airway reflexes |
| |
Many factors can contribute to a reduction in pharyngeal tone, causing collapse and airway obstruction. Residual neuromuscular blockade has been shown to increase the incidence of postoperative respiratory complications. Train-of-four ratios of 0.7–0.9 are associated with impaired pharyngeal function, airway obstruction, and increased risk of aspiration and attenuation of the hypoxic ventilatory response. Reduced laryngotracheal reflexes increase the risk of aspiration and airway soiling. Partial or complete airway obstruction with forceful inspiratory effort generates a significant negative intrathoracic pressure, which opens the oesophagus increasing the risk of regurgitation. The presence of blood in the airway is significant if airway reflexes are obtunded, because the aspiration of blood
clots can cause complete airway obstruction. |
| |
Depletion of oxygen stores at extubation |
| |
Following extubation, the aim is to provide an uninterrupted supply of oxygen to the patient’s lungs. Various factors that contribute to rapid depletion of oxygen stores and a reduction in arterial oxygen Saturation. |
| |
Pathophysiological Causes |
| |
 |
Reduced functional residual capacity Hypoventilation |
 |
Cardiovascular instability |
 |
Diffusion hypoxia |
 |
Neurological dysfunction |
 |
Atelectasis |
 |
Metabolic derangement |
 |
Ventilation ⁄ perfusion mismatch |
 |
Electrolyte disturbances |
 |
Problems related to airway reflexes Shivering |
 |
Airway injury |
|
| |
Pharmacological Causes |
| |
 |
Neuromuscular blocking drugs Opioids |
 |
Residual anaesthetic agents |
|
| |
Human & other factors
|
| |
 |
Inadequate equipment |
 |
Interruption of oxygen supply during |
 |
Inadequate skilled assistance |
 |
patient transfer |
 |
Access to airway e.g. dressings ⁄ gastric tubes ⁄ rigid fixators |
 |
Communication difficulties (e.g. language, mental capacity) |
 |
Patient position |
 |
Removal of oxygen by agitated or uncooperative patient |
|
| |
Airway injury |
| |
Injury to the airway may be the result of direct trauma following surgical or anaesthetic intervention, or it may be indirect due to subsequent bleeding, swelling or oedema. Any surgery or insult in or around the airway can cause problems following extubation. Thyroid surgery, laryngoscopy, panendoscopy, and maxillofacial, cervical spine, carotid and other head ⁄ neck procedures can cause direct airway compromise due to haematoma, oedema, altered lymphatic drainage, vocal cord paralysis and tracheomalacia [54, 55]. Patient position (prone or prolonged Trendelenburg positions), duration of surgery, fluid overload and anaphylaxis may contribute to airway oedema.
|
| |
Anaesthetic airway injury may result from laryngoscopy, or insertion and presence of a tracheal tube or airway adjuncts. Periglottic trauma may result from transoesophageal echocardiography probes and nasogastric tubes, from the use of inappropriately large tube sizes and excessive cuff pressure or from incorrectly positioned tracheal tubes (e.g. with a cuff inflated within the larynx). Problems resulting from airway injury often do not become apparent until after tracheal extubation; direct problems include crico-arytenoid joint dysfunction and vocal cord palsy, and indirect problems may result from pressure effects secondary to haematoma, oedema or mediastinitis . The ASA closed-claims analysis of airway injury during anaesthesia showed that 33% of injuries occurred at the larynx, 19% at
the pharynx, 18% at the oesophagus, 15% at the trachea, 10% at the temporo mandibular joint and 5% at the nose. |
| |
Human factors |
| |
The environment at extubation is not as favourable as at intubation. Equipment, monitoring and assistance may be inadequate. Patient factors contributing to extubation problems may be compounded by distraction, time pressure, operator fatigue, lack of equipment or skilled assistance and poor communication. |
| |
Managing extubation |
| |
There is a lack of compelling evidence to support a ‘one size fits all’ extubation strategy for every patient. There is, however, a general agreement that good preparation is key to successful airway management and that an extubation strategy should be in place for every patient. |
| |
General principles |
| |
Extubation is an elective process, and it is important to plan and execute it well. The goal is to ensure uninterrupted oxygen delivery to the patient’s lungs, avoid airway stimulation, and have a back-up plan, that would permit ventilation and re-intubation with minimum difficulty and delay should extubation fail. Since the introduction of the DAS unanticipated difficult intubation guidelines, the concept of a stepwise approach has been widely accepted. This approach has been used to aid decision making and safe management of extubation. |
| |
The Difficult Airway Society extubation guidelines describe the following four steps: |
| |
Step 1: Plan extubation. |
| |
Step 2: Prepare for extubation. |
| |
Step 3: Perform extubation. |
| |
Step 4: Post-extubation care: recovery and follow-up.
|
| |
Step 1: Plan Extubation |
| |
An outline extubation plan should be in place before induction of anaesthesia and reviewed throughout and immediately before performing extubation. Planning involves an assessment of the airway and general risk factors. The following questions may aid in the decision making process , answers to which will help determine whether extubation is ‘low-risk’ or ‘at-risk’ |
| |
 |
Are there airway risk factors? was the airwaynormal ⁄ uncomplicated at induction? has the airway changed?
|
 |
Are there general risk factors? |
|
| |
Low-risk extubation: |
| |
This is a routine or uncomplicated extubation. The airway was normal ⁄ uncomplicated at induction and remains unchanged at the end of surgery, and no general risk factors are present. |
| |
‘At-risk’ extubation: |
| |
 |
Pre-existing airway difficulties |
 |
Peri-operative airway deterioration |
 |
Restricted airway access |
|
| |
Other things to consider |
| |
Tracheal extubation: awake or anaesthetized? |
| |
When deciding when to extubate, two main considerations should be taken care: |
| |
 |
was there any previous difficulty in controlling the airway?; |
 |
what is the risk of pulmonary aspiration? |
|
| |
As a general rule, patients should be extubated awake. The endpoint for wakefulness in
these studies was taken as swallowing; the incidence of respiratory complications dramatically decreased when extubation was performed when eyes were open, with spontaneous ventilation.
|
| |
Extubation under deep anaesthesia decreases cardiovascular stimulation and reduces the incidence of coughing and straining on the tube. However, the incidence of respiratory
complications has been found to be greater after extubation under deep anaesthesia.
|
| |
Patient position |
| |
Despite advances in anaesthesia, the reported incidence of pulmonary aspiration in the perioperative period has not decreased in the last three decades; it varies from 2.9 to 10.2 per 10 000 anaesthetics.4 Mortality rates of patients who have aspirated vary from 0 to 4.6%.4 The traditional practice of extubating in the left lateral, head-down position maintains airway patency by positioning the tongue away from the posterior pharyngeal wall and also protects the airway from aspiration.
In children, extubation in the recovery position while still anaesthetized is still common practice.
|
| |
Step 2 – Prepare for extubation |
| |
Preparation is aimed at the final optimisation of airway, general and logistical factors to ensure the best possible conditions for success extubation. Together with planning (step 1), preparation (step 2) enables the risk stratification of extubation into ‘low-risk’ and ‘atrisk’ categories, and should always precede extubation (step 3) |
| |
Final evaluation and optimisation of airway factors. The airway should be reassessed at the end of surgery and before extubation. This review should be used to finalise the extubation plan and to determine the most appropriate rescue plan for re-intubation should extubation fail. |
| |
Airway |
| |
It is essential to consider whether bag-mask ventilation would be achievable. Oedema, leeding, blood clots, trauma, foreign bodies and airway distortion can be assessed by direct or indirect laryngoscopy. |
| |
Larynx |
| |
A cuff-leak test may be used to assess subglottic calibre. Clinically, the presence of a large audible leak when the tracheal tube cuff is deflated is reassuring: the absence of a leak around an appropriately sized tube generally precludes safe extubation |
| |
Lower airway |
| |
It is important to consider factors in the lower airway that may contraindicate extubation, such as lower airway trauma, oedema, infection and secretions.
Gastric distension splints the diaphragm and restricts breathing. Gastric decompression with an
oro ⁄ nasogastric tube is advisable if high-pressure facemask ⁄ supraglottic airway ventilation has
been necessary.
|
| |
Final evaluation and optimisation of general factors. |
| |
Neuromuscular block should be fully reversed to maximise the likelihood of adequate ventilation, and restore protective airway reflexes and the ability to clear upper airway secretions. The use of a peripheral nerve stimulator to ensure a train-of-four ratio of 0.9 or above is recommended and has been shown to reduce the incidence of postoperative airway complications.
|
| |
Extubation is an elective process, which should be carried out in a controlled manner with the
same standards of monitoring, equipment and assistance that are available at induction.
Tracheal extubation can take as long to perform safely as tracheal intubation, and this should
be considered when organising list schedules, or sending for the next patient. Communication is
essential, and the anaesthetist, surgeon and theatre team all play an important role.
|
| |
Step 3: Perform extubation |
| |
Step 3 involves the actual performance of extubation
Any extubation technique used should ensure minimum interruption in oxygen delivery to the patient’s lungs.
|
| |
 | Building oxygen stores (pre-oxygenation) |
 | Suction |
 | Alveolar recruitment manoeuvres |
 | Bite block: |
|
| |
Extubation Algorithm |
| |
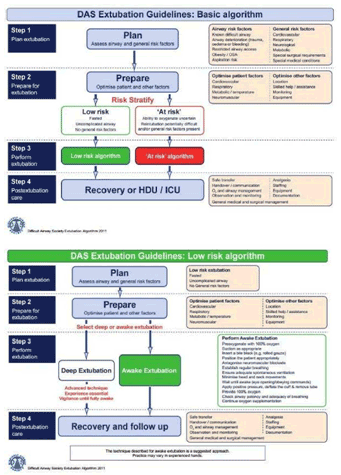
|
| |
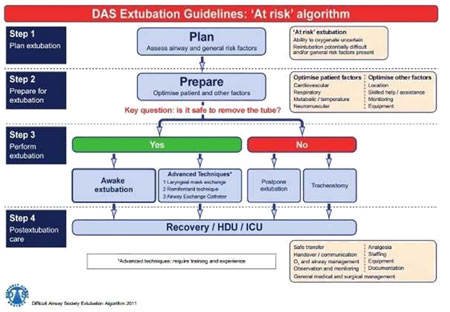
|
| |
Summary:
|
| |
Guidelines are useful in infrequent, life-threatening situations, and have been shown to improve outcomes. Several national guidelines for management of the airway have been published, but none has addressed extubation in detail. Extubation differs from intubation, in that it should always be an elective process with adequate time available to the anaesthetist for methodical management. |
| |
Extubation practice is highly variable, and is not often formally addressed in training. Technical and nontechnical factors can contribute to adverse events at extubation but outcomes are improved by planning, organisation and communication. |
| |
The DAS extubation guidelines promote the concept of an extubation strategy, involving a stepwise approach to planning, preparation and risk stratification, aimed at clear identification and management of patients ‘at risk’ during extubation. |
| |
The evidence base for extubation practice is limited, so inevitably some of the recommendations in these guidelines are based on expert opinion. |
| |
Awake extubation is the preferred technique for most patients. However, deep extubation, laryngeal mask exchange, remifentanil infusion and the use of airway exchange catheters may be beneficial in certain clinical situations. |
| |
Delaying extubation or performing an elective tracheostomy should be considered when it is unsafe to extubate. |