| ANAESTHESIA PEARLS |
- Meet the Machine That Could Replace Anesthesiologists
- The Automatic Lung Parameter Estimator(ALPE)
- ANEclear
| SEDASYS® COMPUTER-ASSISTED PERSONALIZED SEDATION SYSTEM! | ||||||||||||||||||||||||||||||||||||||
| Introduction | ||||||||||||||||||||||||||||||||||||||
|
The SEDASYS® Computer-Assisted Personalized Sedation (CAPS) System is a new method of sedation that integrates physiological monitoring and drug delivery through a computer interface to provide safeguards and facilitate drug titration personalized to the needs of each patient. The SEDASYS System is indicated for the intravenous administration of 1% (10mg/mL) propofol injectable emulsion for the initiation and maintenance of minimal-to-moderate sedation, as defined by the American Society of Anesthesiologists (ASA) Continuum of Depth of Sedation, in ASA physical status I and II patients ≥18 years old undergoing colonoscopy and esophago-gastro-duodenoscopy (EGD) procedures. | ||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Bedside Monitoring Unit (BMU) | ||||||||||||||||||||||||||||||||||||||
| The BMU is connected to the patient in the preprocedure area and stays with the patient through recovery. It provides continuous monitoring of oxygen saturation, blood pressure, and heart rate. | ||||||||||||||||||||||||||||||||||||||
| Procedure Room Unit (PRU) | ||||||||||||||||||||||||||||||||||||||
| The PRU is the primary interface between the physician-led team and the SEDASYS® System and is designed to stay in the procedure room. When the PRU and BMU are connected, the PRU adds capnometry and automated responsiveness monitoring (ARM), oxygen delivery, and delivery of propofol. | ||||||||||||||||||||||||||||||||||||||
| Automated Responsiveness Monitoring (ARM) | ||||||||||||||||||||||||||||||||||||||
|
The ARM allows the System to gauge the depth of propofol sedation by measuring patient responsiveness. The ARM provides the patient with a combined auditory request ("squeeze the handset") through an earpiece and tactile stimulus (the handset vibrates). When the patient responds by squeezing the handset, signals are transmitted to the System, and the time it took the patient to respond is displayed to the physician. The stimuli occur at one of three increasing levels, depending on the rapidity of the patient's response. The stimuli start off with a soft voice and a mild vibration that escalate twice over 14 seconds. The third auditory request is loud, with the handset vibrating vigorously. If there is no response in 14 seconds, the patient is deemed nonresponsive to the ARM. Note that nonresponsiveness to the ARM does not mean the patient has transitioned to a deeper-than-intended level of sedation. NOTE: If a patient is hearing impaired or physically unable to squeeze the ARM handset, the procedure can be conducted in Clinician Response Mode. In this mode, the System does not transmit stimuli to the patient. Instead, the System prompts the clinician at regular intervals to assess responsiveness by asking "Is the patient responsive?" The clinician must select Yes or No. If the clinician does not answer the question within 14 seconds, the System will record the patient as being nonresponsive. | ||||||||||||||||||||||||||||||||||||||
| Propofol Delivery Cassette | ||||||||||||||||||||||||||||||||||||||
| The Drug Delivery Cassette is a proprietary component of the System, when placed within the PRU, enables the delivery of propofol from the drug vial. | ||||||||||||||||||||||||||||||||||||||
| Display Monitor on the Procedure Room Unit (PRU) | ||||||||||||||||||||||||||||||||||||||
| The user interface on the PRU displays comprehensive physiological and responsiveness monitoring data. | ||||||||||||||||||||||||||||||||||||||
| The Display Monitor has a touchscreen that displays patient data and allows the clinician to interact with the PRU. | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Patient Alarms – Detect, Alert and Respond to Adverse Patient Physiology | ||||||||||||||||||||||||||||||||||||||
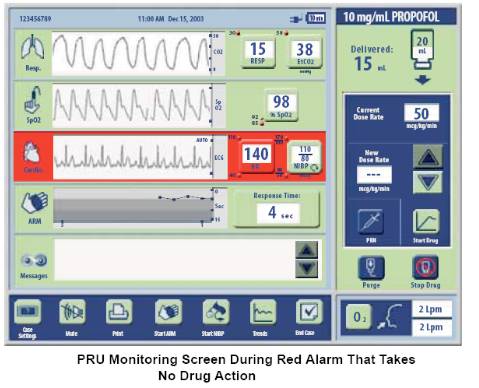
| The System has both yellow and red alarms. Red alarms trigger in response to physiologic conditions such as hypoxemia, apnea, bradycardia, tachycardia, hypotension, hypertension and high respiratory rate. The following graphic is an example of a red alarm for tachycardia that would present to the physician-led team. This would be accompanied by a distinct audible tone. | ||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
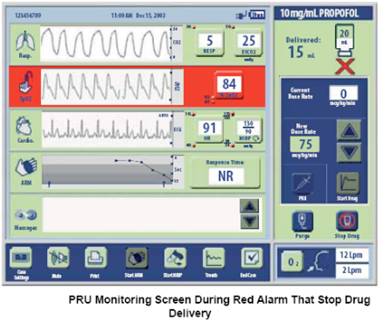
| For physiological conditions that have a high correlation with over-sedation, hypoxemia and prolonged apnea, the System's red alarms stop delivery of propofol and instructs the patient to take a deep breath. After this red alarm clears, the clinician must manually restart the delivery of propofol. The graphic below shows the System during a red alarm for hypoxemia. | ||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
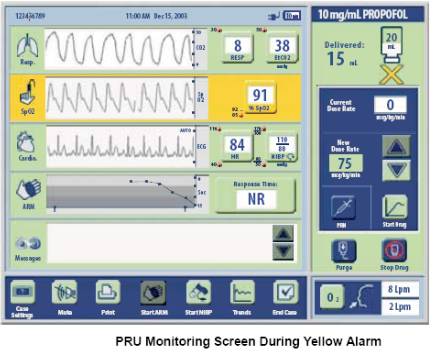
| Yellow Alarms are designed to guard against over-sedation related conditions such as hypoxemia and prolonged apnea. They trigger on more conservative thresholds compared to red alarms; for example SpO2 < 92%. The System will automatically respond by suspending drug delivery and instructing the patient to take a deep breath. Once the yellow alarm clears, the System will automatically resume drug delivery at an appropriately reduced rate. The following graphic shows the System during a yellow alarm for hypoxemia. This is accompanied by an audible tone, different than the red alarm tone. | ||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
| Propofol Delivery Cassette | ||||||||||||||||||||||||||||||||||||||
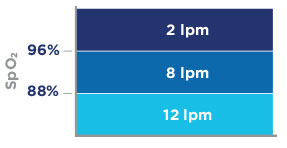
| Oxygen is delivered to the patient's nose and mouth and is automatically started upon detection of respiration. The amount of oxygen delivered, measured in liters per minute (lpm), is automatically increased as patient's SpO2 decreases per the graphic below. Since the integration relies on the presence of oxygen, an oxygen source must be connected to the SEDASYS® System. The System will not deliver propofol without supplemental oxygen delivery. | ||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
| Indications | ||||||||||||||||||||||||||||||||||||||
| The SEDASYS® System is indicated for the intravenous administration of 1% (10 mg/mL) propofol injectable emulsion for the initiation and maintenance of minimal-to-moderate sedation, as defined by the American Society of Anesthesiologists (ASA) Continuum of Depth of Sedation, in ASA physical status I and II adult patients undergoing colonoscopy and esophagogastroduodenoscopy (EGD) procedures. | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Anesthesia Professionals Question Safety of Non Experts Giving Propofol to Patients via New Sedasys Machine | ||||||||||||||||||||||||||||||||||||||
|
Right now, only four U.S. hospitals are using the Sedasys anesthesiology machine to sedate patients before surgery. Johnson & Johnson has been cautiously rolling out the machine after winning approval from the Food and Drug Administration in 2013. The FDA originally rejected the machine in 2010, but later approved after Johnson & Johnson agreed it would only be used for simple screenings--like colonoscopies or endoscopies--and only when an anesthesiology doctor or nurse was on-call. A 2004 joint position statement of the AANA and the American Society of Anesthesiologists (ASA) concurs, saying, "Whenever propofol is used for sedation/anesthesia, it should be administered only by persons trained in the administration of general anesthesia, who are not simultaneously involved in these surgical or diagnostic procedures." Even the FDA-approved labeling on propofol warns that the drug should only be provided by persons qualified in general anesthesia, because the drug's effects cannot be reversed. According to Johnson & Johnson, facilities where the Sedasys is used should have an anesthesia professional immediately available for assistance or consultation. Johnson & Johnson also stresses the need for the propofol provider to be trained in dealing with the drug's cardiorespiratory effects—abilities that CRNAs and anesthesiologists master during years of advanced education and clinical training. | ||||||||||||||||||||||||||||||||||||||
| Would you put your life in the 'hands' of this machine? | ||||||||||||||||||||||||||||||||||||||
| The Automatic Lung Parameter Estimator (ALPE) is an essential Clinical Diagnostic Pulmonary Device for Anaesthesiologists | ||||||||||||||
|
Traditional methods of diagnostic a full pulmonary function are time-consuming and costly. In addition, these methods do not give adequate insight in the patient's pulmonary gas exchange capabilities. Moreover, providing only partial insight, these methods may lead to false assessments of surgical complications. ALPE essential is the first and only product that lets you perform accurate, cost-effective characterization of pulmonary gas exchange – with one non-invasive measurement in less than 8 minutes. | ||||||||||||||
 |
||||||||||||||
|
Most traditional pulmonary procedures and tests are limited to assessing whether the patient is operable, and do not give insight into the patient's oxygenation capabilities. Nevertheless, such preoperative insight is crucial when dealing with patients with shortness of breath or patients undergoing complex surgery. With ALPE essential, you can perform targeted preoperative treatment based on the patient's pulmonary gas exchange expressed as O2 –loss and Shunt with the purpose of minimizing perioperative and postoperative oxygenation issues. | ||||||||||||||
| ALPE essential – non-invasive measurement of pulmonary gas exchange expressed as O2-loss and Shunt. | ||||||||||||||
 |
||||||||||||||
|
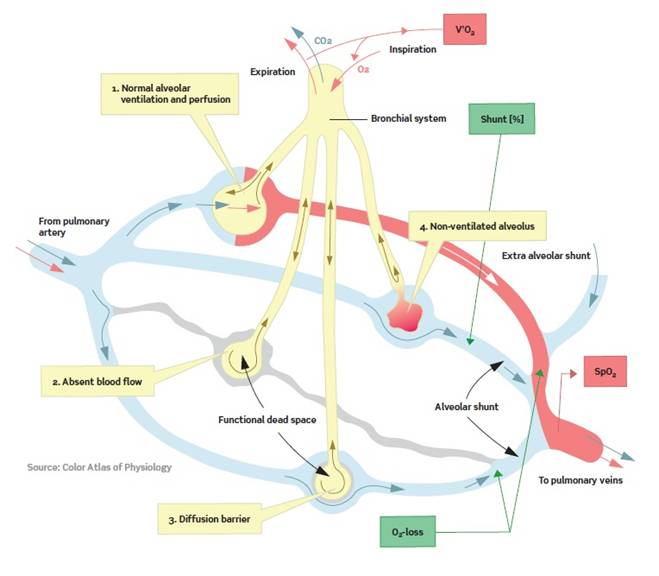
The principles for pulmonary gas exchange are well-known, but gas exchange is dependent on many varying factors such as insufficient ventilation, shunting of blood, ventilation-perfusion mismatch, abnormal diffusion capacity, cardiac output, and acid-base state of the arterial blood. Full understanding of an oxygenation problem therefore requires complex measurements which have been impractical with today's technology in daily clinical use. ALPE essential is the clinical solution. The traditional ways to treat hypoxemic patients are supplementary oxygen, diuretics or by alveoli recruitment techniques such as for example CPAP. | ||||||||||||||
 |
||||||||||||||
|
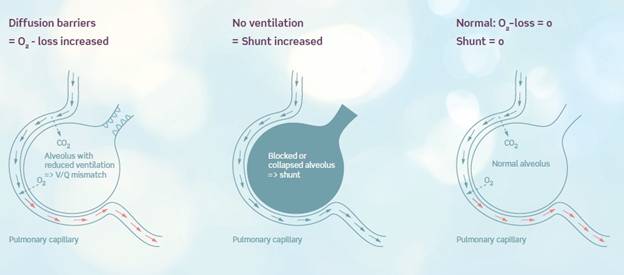
ALPE essential is based on a mathematical model, which from breath-by-breath analysis and measurements of expired end-tidal oxygen (FetO2) and arterial saturation (SpO2) provides health care professionals with two parameters giving reliable pulmonary gas exchange insight. The measurements are non-invasive and can be done bedside in app. 5-8 minutes. The staff utilization of the system is user-friendly by a simple and self-explaining interface. ALPE essential ascribes hypoxemia to ventilation-perfusion mismatch and pulmonary shunt. Gas exchange impairment is thereby divided into a part that can be treated with oxygen therapy and a part which will not respond to extra oxygen, respectively. The method consists of solving a mathematical gas exchange model by making the best fit to the measured end-tidal expiratory oxygen (FetO2) and arterial saturation (SpO2) levels. As a result two clinical parameters are presented: Ventilation-perfusion mismatch as quantified by the first parameter, O2 - loss which is the extra oxygen that the patient requires. The second parameter is the pulmonary shunt. Completely blocked alveoli result in perfused but not ventilated areas of the lung. In these areas no gas exchange can happen. It has e.g. been shown that the area of the atelectatic lung region correlates significantly with increased shunt. The patient breathes through a mask coupled to a respiratory unit. Arterial oxygen saturation is monitored with a built-in pulse oximeter, and expired oxygen is measured with an oxygen and flow sensor. The gas exchange is characterized via the patented ALPE algorithm and expressed as O2-loss and Shunt. The whole procedure is non-invasive and takes less than 8 minutes and can be performed bedside sparing the patient and clinician any discomfort or stress. | ||||||||||||||
 |
||||||||||||||
| The APLE Curve | ||||||||||||||
| The ALPE technology is built into the ALPE essential device, which is working by complex software that integrates data measured from the patient. These data are: | ||||||||||||||
|
||||||||||||||
|
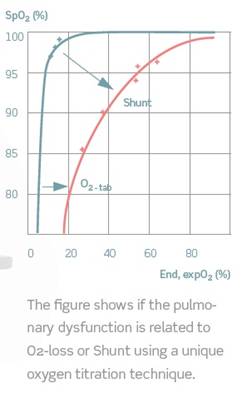
The gas exchange impairment parameters estimated are the O2-loss, which is a measure of the level of extra oxygen pressure needed to reach normal saturation and the Shunt, which is the percentage of blood not reaching the alveoli. The values, O2-loss and Shunt, are estimated by a patented mathematical model fitted to the points of the measured arterial saturation and expiratory oxygen. As illustrated on the figure below the O2-loss value can also be interpreted as the right-ward shift of the curve given by the end tidal expired oxygen fraction versus the arterial saturation. The Shunt value can equally be interpreted as the down-ward shift of this curve. | ||||||||||||||
 |
||||||||||||||
|
The figure shows if the pulmonary dysfunction is related to O2 - loss or shunt using a unique oxygen titration technique. The blue curve shows a normal situation where a rise in oxygen percentage above atmospheric air gives 100% saturation. When a drop in oxygen percentage is present the drop in saturation will be minor. The red curve is from a patient with both shunt and a O2 - loss. An increase of the oxygen percentage above atmospheric air does not increase the saturation due to the blocked alveoli. A minor decrease of the oxygen percentage on the other hand gives a rapid decrease in saturation causing the curve to make a rightwards shift. This indicates a O2 - loss With the ALPE system a nurse can do the measurements in the ward. The interpretation of the results is easy, straightforward, and leads to decision on the future treatment. | ||||||||||||||
| Minimize and document pulmonary complications during the anaesthesia process | ||||||||||||||
 |
||||||||||||||
| ALPE essential lets you perform serial measurements and document everything in one single report. This report is the perfect starting point for documenting the anaesthesiologist's perioperative and postoperative focus on, and treatment of, the patient's oxygenation capacity. | ||||||||||||||
| What Experts Say | ||||||||||||||
 "The Automatic Lung Parameter Estimator (ALPE) technology represents such a compromise between feasibility and complexity," says Karbing. First ventilator flow, gasses and pulse oximetry are measured with varying levels of inspiratory oxygen. Then, a two-parameter model of pulmonary gas exchange is fitted to measurements, and the resulting model fitted parameters, pulmonary shunt and O2-loss are output. These parameters represent the major factors causing pulmonary gas exchange problems: shunting of venous blood and lung regions with low ventilation-perfusion (V/Q) ratios. "The gas exchange model used in ALPE has previously been successfully compared with the experimental golden standard, the MIGET, indicating that the ALPE parameters are physiologically plausible," says Karbing, who adds that the technology is commercially available in Europe.He has presented two applications of ALPE at the bedside: the setting of inspiratory oxygen and monitoring the effects of positive end-expiratory pressure (PEEP). For setting inspired oxygen, the O2-loss parameter specifies the additional percentage of inspiratory oxygen necessary at the mouth to secure normal oxygenation of that which is 'oxygen responsive'. The physiological rationale behind this is that problems with low V/Q can be countered by increasing inspiratory oxygen, whereas regions with shunt problems are not oxygen responsive. "For monitoring the effects of PEEP, we know from studies with Multiple Inert Gas Elimination Technique (MIGET) that changing PEEP modifies V/Q distributions in the lung, and I can illustrate use of ALPE in this context through clinical cases," he concludes.
"The Automatic Lung Parameter Estimator (ALPE) technology represents such a compromise between feasibility and complexity," says Karbing. First ventilator flow, gasses and pulse oximetry are measured with varying levels of inspiratory oxygen. Then, a two-parameter model of pulmonary gas exchange is fitted to measurements, and the resulting model fitted parameters, pulmonary shunt and O2-loss are output. These parameters represent the major factors causing pulmonary gas exchange problems: shunting of venous blood and lung regions with low ventilation-perfusion (V/Q) ratios. "The gas exchange model used in ALPE has previously been successfully compared with the experimental golden standard, the MIGET, indicating that the ALPE parameters are physiologically plausible," says Karbing, who adds that the technology is commercially available in Europe.He has presented two applications of ALPE at the bedside: the setting of inspiratory oxygen and monitoring the effects of positive end-expiratory pressure (PEEP). For setting inspired oxygen, the O2-loss parameter specifies the additional percentage of inspiratory oxygen necessary at the mouth to secure normal oxygenation of that which is 'oxygen responsive'. The physiological rationale behind this is that problems with low V/Q can be countered by increasing inspiratory oxygen, whereas regions with shunt problems are not oxygen responsive. "For monitoring the effects of PEEP, we know from studies with Multiple Inert Gas Elimination Technique (MIGET) that changing PEEP modifies V/Q distributions in the lung, and I can illustrate use of ALPE in this context through clinical cases," he concludes.
|
||||||||||||||
| Reference: | ||||||||||||||
|
S.E. Rees, S. Kjærgaard, P. Thorgaard, J. Malczynski, E. Toft, S. Andreassen. The Automatic Lung Parameter Estimator (ALPE) system: Non-invasive estimation of pulmonary gas exchange parameters in 10-15 minuts. Journal of Clinical Monitoring and Computing, 17:43-52, (2002). S. Kjærgaard, S.E. Rees, J. Malczynski, J. Ahrenkiel Nielsen, P. Thorgaard, E. Toft, S. Andreasen. Non-invasive estimation of shunt and ventilation-perfusion mismatch. Intensive Care Med, 29:727-734, (2003). S. Kjærgaard, S.E. Rees, J. Grønlund, E.M. Nielsen, P. Lambert, E. Toft, S. Andreassen. Hypoxaemia after cardiac surgery: clinical application of a model of pulmonary gas exchange. European Journal of Anaesthesiology, 21:296-301, (2004). S. Kjærgaard, S.E. Rees, J.A. Nielsen, M. Freundlich, P. Thorgaard, S. Andreassen. Modelling of hypoxaemia after gynaecological laparotomy. Acta Anaesthesiologica Scandinavica, 45:349-356, (2001). L. Tokics, G. Hedenstierna, L. Svensson, B. Brismar, T. Cederlund, H. Lund-quist, Å. Strandberg. V/Q distribution and correlation to atelectasis in anesthetized paralyzed humans. Journal of Applied Physiology, 81:1822-1833, (1996). S.E. Rees, S. Kjærgaard, S. Andreassen, G. Hedenstierna. Reproduction of MIGET retention and excretion data using a simple mathematical model of gas exchange in lung damage caused by oleic acid infusion. Journal of Applied Physiology. 101:826-832, (2006). B.W. Smith, S.E. Rees, D.S. Karbing, S. Kjærgaard, S. Andreassen. Quantitative assessment of pulmonary shunt and ventilation–perfusion mismatch without a blood sample. Conference Proceedings, IEEE Eng Med Biol Soc. 2007, 2007:4255-8. B.S. Rasmussen, J. Sollid, S.E. Rees, S. Kjærgaard, D. Murley, E. Toft. Oxygenation within the first 120h following coronary artery bypass grafting. Influence of systemic hypothermia (32°C) or normothermia (36°C) during the cardiopulmonary bypass: a randomized clinical trial. Acta Anaesthesiologica Scandinavica, 50: 64-71, (2006). | ||||||||||||||
| Introduction | ||||||||||||||||||||||||||||||||
|
The ANEclearTM (formerly the QED-100) is an inexpensive, easy-to-use, disposable device that quickly and actively removes unwanted inhaled anesthetics following surgery. The ANEclear works easily with an endotracheal tube, or an LMA with spontaneously breathing patients under isoflurane, sevoflurane or desflurane to quickly and actively remove unwanted residual agents at the end of surgery. | ||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
| Rapidly removing residual inhaled anesthetics at the end of surgery reduces the effects these agents have on patients at risk for PONV and OSA. The ANEclear reduces risk and improves life for your challenging patients. | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
When general inhaled anesthesia is administered, anesthetics in the form of vapors are delivered to a patient in .5% to 10% concentrations, depending on the anesthetic agent used. As the individual inhales an anesthetic vapor, the vapor enters the lungs, moves into the bloodstream and is carried by the blood to the brain. Once the surgical procedure has been completed, the process is reversed. Blood carries the anesthetic from the brain to the lungs where it is exhaled from the body. The time it takes for a patient to awaken after general anesthesia depends on the time it takes to remove the anesthetic from the brain. The concentration of anesthetic in the patient's brain needs to decrease to the level at which consciousness returns. The higher the brain blood flow and the ventilation rate, the quicker the effects of the general anesthetic are reversed and the quicker the patient wakes up. Time to emergence from inhaled volatile anesthesia is limited by the time required to remove anesthetic gases from the lungs, and the time required to carry anesthetic agents from the brain via the blood stream to the lungs. The classic approach to accelerating the clearance of anesthetic gasses from the lungs is to artificially raise ventilation. Raising ventilation lowers alveolar carbon dioxide partial pressure and thus arterial carbon dioxide partial pressure. This has the undesired effect of lowering blood flow to the brain and delaying emergence from anesthesia. Herein lies the problem; increased ventilation will speed removal of anesthetic from the lungs and arterial blood; however, increased ventilation will also decrease blood flow to the brain. Decreased ventilation will allow increased blood flow to the brain, but the concentration of anesthetic in the blood will remain high, thereby eliminating the benefit of higher brain blood flow. Anecare has created the ANEclear, a unique patent-pending disposable device that allows the effect of increased ventilation to work without lowering blood CO2 concentration which leads to lower blood flow to the brain. When the device is activated in the anesthesia breathing circuit, the fraction of inspired agent is essentially zero and patient ventilation can be increased without an undesirable decrease in blood flow to the brain. By creating this state of mild hypercapnia combined with hyperventilation, the ANEclear actively eliminates anesthetic from the patient and increases spontaneous respiration. The result is a patient who can be extubated sooner and who is more awake at extubation in the OR. Further, the ANEclear continues to accelerate spontaneous respiration following extubation, which means patients are more alert and conscious in the PACU and can participate in pain assessment and management. This translates into improved patient safety, satisfaction and a shorter PACU stay. | ||||||||||||||||||||||||||||||||
| Aneclear Demo Video | ||||||||||||||||||||||||||||||||
