Conference Lectures
Coagulation disorders in End Stage Liver Disease
The majority of patients with end-stage liver disease(ESLD) have complex and multifactorial alterations that involve all of the components of hemostasis.
Thrombocytopenia is a frequent finding, with platelet counts usually ranging between 30-100 x109/L.Associated defective platelet aggregationreflects why BT is often more prolonged than would be predicted from the degree of thrombocytopenia. Although anemia coexists in a majority of these patients, but unlike uremia anemia does not seem to play a role in causing the hemorrhagic tendency in liver disease. Screening tests of the coagulation phase of hemostasis (ie, PT, aPTT) are consistently prolonged becausemultiple coagulation factors are low in plasma in proportion to the degree of compromised protein synthetic capacity of the liver. Fibrinolyis is commonly seen in cirrhosis on the basis of the increase of tissue plasminogen activator(tPA) and decrease of the naturally occurring inhibitors of plasmin.
Hemostasis abnormalities in cirrhosis
Hemostasis component |
Antihemostatic abnormalities |
Primary hemostasis |
Thrombocytopenia, defective platelet aggregation on light transmission aggregometry |
Secondary hemostasis |
Low procoagulant factors (fibrinogen, prothrombin, factors V, VII, IX, X, XI, XIII) |
Fibrinolysis |
High plasminogen activator, low plasmin inhibitors |
These multiple and complex alterations of laboratory methods have traditionally led the clinician to infer that hemostasis is globally impaired in cirrhosis.
Bleeding complications in patients with liver disease
- Chronic Liver Disease
The most frequent bleeding complication in patients with cirrhosis is upper GI bleeding secondary to variceal rupture.Unlike traditional teaching it is now known that variceal bleeding depends more on local vascular abnormalities and portal hypertension causing increased vascular pressure rather than on abnormalities of hemostasis as reflected by deranged tests of coagulation.
Other bleeding complications, including bruising, purpura, epistaxis, gingival bleeding, menorrhagia, and bleeding associated with invasive procedures may be related to defective hemostasis.
Factors which increase the risk of bleeding in patients with ESLD are-
- Bacterial infection
Bacterial infection in patients with cirrhosis is associated with increased mortality and GI bleeding. Up to two-thirds of patients with gastrointestinal bleeding have a bacterial infection. TEG studies have shown a hypocoagulable state in cirrhotic patients with an active infection, and this has been associated with an increased bleeding risk. Increased circulating heparin-like molecules are in part responsible for this hypocoagulable state. Prophylactic administration of antibiotics has been shown to reduce both mortality and bleeding risk.
- Renal failure
Renal Dysfunction frequently complicates liver disease, and aggravates hemostatic abnormalities. Uremia is associated with disturbed platelet-vessel wall interaction mediated by multiple mechanisms. Renal function is an important predictor of intraop blood loss and transfusion requirements in patients undergoing Liver Transplantation(LT).
Clinical evidence of hypercoagulation
Hypercoagulability in these patients is often overlooked. Patients with liver disease do develop deep vein thrombosis and pulmonary embolism at appreciable rates (between 0.5% and 1.9%).All the three components of Virchow’s triad- hemodynamic changes, damaged vasculature and hypercoagulability are often present in patients with liver disease and contribute to thrombosis risk.Thrombosis of the portal and mesenteric veins is common in patients with advanced cirrhosis. Hemodynamic changes decreased portal flow and inherited thrombophilia ie factor V Leiden deficiency; prothrombin G20210A mutation etc may both play a role. Hypercoagulation may have a role in the development of Hepatic artery thrombosis after liver transplantation (traditionally considered a postop surgical complication).
Conventional Tests of Coagulation and Risk of bleeding in Liver Disease
Bleeding time (BT) is prolonged in cirrhosis and has been used to measure primary hemostasis and reflects a deficit in the vasoconstrictor response also. Correction of BT has not correlated with decreased bleeding in various studies.
Conventional tests of the clotting cascade such as the PT and APTT correlate poorly with procedure-related bleeding in patients with cirrhosis. This lack of predictive power can best be explained by deficiency of the naturally occurring anticoagulants protein C, antithrombin and TFPI that are reduced in parallel with procoagulant factors. Protein C in vitro is activated poorly without thrombomodulin (absent in usual clinical assays) and therefore cannot exert its full anticoagulant activity.
Accelerated Intravascular Coagulation and Fibrinolysis (AICF)
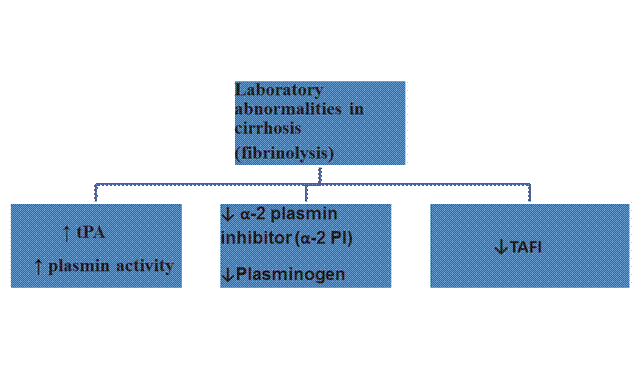 Laboratory abnormalities in decompensated cirrhosis come to resemble disseminated intravascular coagulation (DIC). Distinguishing features of this state Accelerated Intravascular Coagulation and Fibrinolysis (AICF)from true DIC are-
Laboratory abnormalities in decompensated cirrhosis come to resemble disseminated intravascular coagulation (DIC). Distinguishing features of this state Accelerated Intravascular Coagulation and Fibrinolysis (AICF)from true DIC are-
- Relatively stable platelet levels
- High factor VIII levels
- Absence of uncompensated thrombin generation
- Absence of classical end organ damage from intravascular coagulation
- Subtle forms of micro thrombotic disease
Rebalanced hemostasis in liver disease
Patients with liver disease may experience both bleeding complications and thrombotic episodes. As PT/ APTT are not sensitive for deficiencies of the anticoagulants, these tests only reflect the deficiencies in pro coagulant proteins. Normal thrombin generation in the presence of thrombomodulin in plasma from these patients suggests a rebalanced hemostasis despite prolongation of PT and APTT.But therebalanced hemostatic status of patients with ESLD is unstable and fragile.
Therapeutic implications
- Abnormal hemostasis tests in patients with liver disease are thus not indicative of a bleeding tendency.
- Efficacy of FFP and platelet concentrate infusion to avoid bleeding has never been demonstrated.
- Complete normalization of laboratory parameters in cirrhotic patients is rarely achieved by administration of platelet concentrates.
- The common clinical practice to correct abnormal hemostasis before invasive procedures is to fulfill local general clinical guidelines but these guidelines do not always specifically comment on the liver disease patient.
Over the years a steady decline in transfusion requirements has been observed liver transplantation (even being offered to Jehovah’s witnesses now). The approach to start surgery without correction of abnormal hemostasis tests by FFP and platelet concentrates and real time monitoring of coagulation using Point of Care monitoringdevices (TEG) has actually contributed to this decline.Experience from LT surgery clearly demonstrates that preoperative correction of these laboratory abnormalities does not reduce, and may in fact promote, bleeding. A conservative transfusion policy reduces overall periop transfusion requirements by avoiding fluid overload.
Balance of hemostasis in ESLD
.
Coagulation disorders in End stage Kidney Disease
CHRONIC KIDNEY DISEASE AS A PROCOAGULANT STATE
End-stage renal disease (ESRD) is a prominent and much feared complication of the CKD, but the high mortality rate seen in CKD is mainly due to increased incidence of cardiovascular disease. This is secondary to a greater prevalence of traditional cardiovascular risk factors such as older age, smoking, hypertension, Type II DM and obesity (all considered prothrombotic conditions) than the general population.
Hemostatic abnormalitiesin CKD patients

The factors above shift the balance of hemostasis in the favor of coagulation and thrombosis, increasing the risk of atherosclerosis and thrombotic events. Increased fibrinogen concentration may contribute to atherosclerotic plaquegrowth by increasing plasma viscosity, promoting plateletaggregation, and inducing regional fibrin depositionin the injured endothelium. Patients with CKD have increased Tissue Factor levels which has been shown to be an inflammatory mediator may contribute to the development of atherosclerosis in CKD patients.
The procoagulant state in patients with hypertension has been well documented to be associated with activation of the renin-angiotensin-aldosteronesystem (RAAS). Pharmacologicalinhibition of the RAAS in CKD patients has been associated with a reduced CVS morbidity and mortalityand with aslower progression of the underlying kidney diseasein these patients.
END-STAGE RENAL DISEASE AND INCREASED RISK OF BLEEDING
Patients with uremic bleeding typically present with ecchymosis, purpura, epistaxis and bleeding from venipuncture sites. These patients can also present with gastrointestinal or intracranial bleeding.As CKD advances, the procoagulant abnormalities persist, but in addition, patients start to exhibit platelet dysfunction (Qualitative) that typically manifests with an increased risk of cutaneous, mucosal, or serosal bleeding.
Platelet dysfunction in patients with advanced CKD is secondary to cumulative effects of multiple platelet functional abnormalities and faulty arachidonic acid and prostaglandin metabolism. These lead to impaired platelet adhesion and aggregation. Certain uremic toxins such as guanidinosuccinic acid and methyl guanidine may contribute to platelet dysfunction by stimulating NO release.Correction of anemia improves platelet function in these patients suggesting some pathogenic role in development of platelet dysfunction
Hemostasis defects in uremia
Patients with CKD commonly have prolonged bleeding time (BT). This test is a reflection of ex vivo measurements of defective platelet interactions with the vessel wall and the delayed formation of the primary hemostatic plug.
Thrombocytopenia is a rare cause of prolonged BT in uremia. Biochemical platelet alterations and the resulting defects of aggregation slow the formation of the primary hemostatic plug. But it is the impaired platelet adhesion to injured vessels that is the main determinant of the prolonged BT in uremia.
Etiologies of platelet dysfunction in patients with renal disease
Platelet defects |
Defects of platelet adhesion to the vessel wall |
Medication-induced defects of platelet function |
Light transmission aggregometry defects |
Anemia |
Antiplatelet agents |
Decreased TXA2 formation |
VWF dysfunction |
NSAID’S |
Low content of serotonin and ADP |
Uremic toxins |
beta-lactam antibiotics |
Impaired Ca2 dependent functions |
Enhanced nitric Oxide production |
third-generation cephalosporins |
Impaired release of alpha- and dense-granule contents |
Impaired function of GPIIb/IIIa |
|
VWF dysfunction has been implicated as an important cause in bleeding disorders in CKD patients, as both cryoprecipitate and desmopressin improve the BT in uremia. Enhanced endogenous production of NO (a vasodilator and platelet function inhibitor) has also been postulated to play a contributory role. However, Anemia a constant feature in advanced ESRD is now considered a critical determinant of defective platelet adhesion to the vessel wall and the resulting prolongation of BT.
It has been consistently been shown by various investigators that the BT is prolonged in proportion to the degree of anemia in uremic patients, and that BT is shortened or corrected when the Hct.is increased to ≥ 30% by RBC transfusion. The Hct. should be kept at approximately 30%, as higher values and full correction of anemia increase the risk of thrombotic complications, particularly myocardial infarction and ischemic stroke.
The mechanisms involved in improvement in platelet adhesion by correction of anemia are-
- Increased number RBCs in the circulation push more platelets and leucocytes from the axial center of flowing blood toward the periphery, thereby enhancing platelet contact with the vessel wall and formation of the primary hemostatic plug.
- Release from RBCs of ADP, a powerful inducer of platelet aggregation.
- Scavenging effect exerted by hemoglobin on nitric oxide
The coagulation phase of hemostasis, as explored by such screening tests as activated APTT and PT is usually normal in uremic patients.
Therapeutic control of bleeding in uremia
- Erythropoietin
- Dialysis
- Cryoprecipitate (CPP)
- Desmopressin
Summary
Patients with ESLD and ESRD have multiple and complex defects of coagulation systems. The assessment of risk of bleeding in these patients requires a clear understanding of the pathophysiological processes involved. Use of newer monitoring techniques of primary (PFA-100) and secondary hemostasis-TEG can help to evaluate risk of bleeding and guide therapy.